Hot flash - menopause news doesn't tell the whole story
FDA removes Black Box warning from Hormone Relief Therapy packages. Why?
A few decades ago, the Outraged Consumer was between journalism gigs and took a pay-the-rent position at an influential organization that took in millions of dollars yearly to support its self-proclaimed mission of promoting “Vital Aging.”
The organization ran various educational and training programs for seniors and caregivers, many of them sponsored by government agencies and large corporations. Big Pharma was one of the group’s major Sugar Daddies, underwriting what were called “independent research studies” that — what a surprise — just happened to support the business interests of the sponsor.
One of the larger such contracts was for hormone replacement therapy (HRT), a witches’ brew of drugs that supposedly helped women tolerate the annoying symptoms of menopause.
The Outraged Consumer soon found himself in a conference room where he was told his job was to take careful notes as a representative of the drug company spelled out what the HRT study it was funding should find and what points should be emphasized. After he asked a question or two, he was told to stop interrupting and do as he was told.
From this humble beginning, an impressive-looking study was pasted together and “educational campaigns” were organized, targeting doctors, women and the media, spreading the party line that HRT was the key to maintaining a youthful and healthy lifestyle well into a woman’s later years.
No one in the aging organization questioned this, objected to it or even asked any questions about it, other than when it would be ready and how much revenue it would bring in.
This is the template that is routinely followed when drug companies, insurers, healthcare organizations and other big players want to push a particular product line. The goal is first to indoctrinate doctors in the alleged benefits of the product so that they will be inclined to prescribe it to patients. The secondary goal is to spread the same line to consumers, the media and anyone else who plays a part in keeping the revenue funnel full.
Relief but at what cost?
The HRT campaign went smashingly well and was eagerly adopted by women seeking at least fleeting relief from the routine ravages of aging. But as time went by, studies found a disturbing correlation between the use of HRT and the incidence of various cancers as well as strokes and heart attacks.
The Food and Drug Administration (FDA) took note of this and eventually ordered a Black Box warning on HRT products. That’s the strictest warning it can issue. It’s equivalent to putting a skull and crossbones on a label.
HRT sales flat-lined and stayed that way until recently, when news stories planted by P.R. log-rollers started appearing here and there that implied the FDA had acted hastily and that women were suffering as a result.
Just coincidentally — and, of course, we’re simply saying that there was correlation but no proven causation — Robert F. Kennedy Jr., a lawyer with no formal training in medicine, became the secretary of FDA’s parent agency, the Department of Health and Human Services.
This brings us to November 10, 2025, when FDA announced it was removing the Black Box warning, calling it “historic action to restore gold-standard science to women’s health,” saying the action followed “more than two decades of fear and misinformation.”
“Today, we are standing up for every woman who has symptoms of menopause and is looking to know her options and receive potentially life-changing treatment,” said Kennedy. “For more than two decades, bad science and bureaucratic inertia have resulted in women and physicians having an incomplete view of HRT. We are returning to evidence-based medicine and giving women control over their health again.”
On the other hand …
Or could it be that with a new sheriff in town, Big Pharma and its accomplices were able to once again put their spin on the matter?
Nina Zeldes, PhD, a health researcher at Public Citizen’s Health Research Group, took a dim view of the FDA’s claims.
“In fact, the opposite is true. These treatments have well documented cardiovascular, cognitive and cancer safety risks,” she said. “Overblown press conference announcements and unsubstantiated labeling updates do not protect women’s health. The FDA should advance women’s health by making decisions based on high-quality data from long-term randomized controlled trials and the formal advisory committee process.
“Instead of making unsubstantiated claims about improving women’s health, the FDA should encourage or fund research that establishes the benefits and risks of short-term and long-term use of hormone replacement therapy,” Zeldes said.
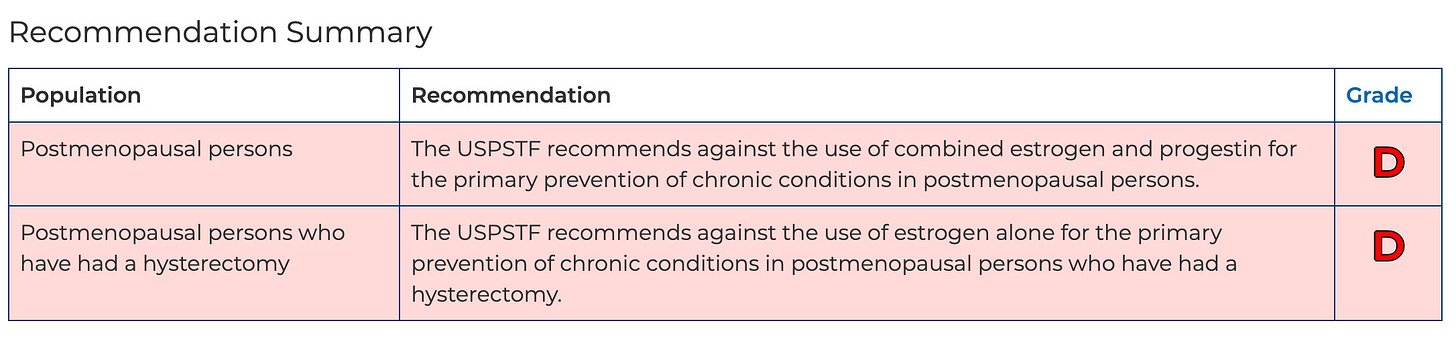
Zeldes noted that the U.S. Preventive Services Task Force has recommended against hormone replacement therapy for the prevention of chronic conditions in post-menopausal women.
“Instead of making unsubstantiated claims about improving women’s health, the FDA should encourage or fund research that establishes the benefits and risks of short-term and long-term use of hormone replacement therapy,” Zeldes said.



The tone of your story is very critical and condescending. “Meno-shaming” if you will. Not one of the women I know who have gone on HRT used it for "fleeting relief from the routine ravages of aging." On the other hand, I don't know any Kardashians.
Like every other woman I know, I went on HRT (the lowest dose that could be prescribed) to deal with debilitating hot flashes, which I am firmly convinced are the basis for the old stories of spontaneous combustion. That’s how you feel with a hot flash, like someone just lit you on fire. I tried black cohosh and all the other natural remedies, and they didn’t work for me. I knew the risks of HRT, but I weighed quality of life against quantity of life. I stayed on the drugs for several years, then weaned myself off. That was 20 years ago.
To be clear, I am not advocating for removal of the black box. I think that physicians need to clearly explain the risks to patients, and it is their responsibility to answer questions and be reasonably certain that their patients understand the risk. The black box, should anyone actually read it, reinforces these risks.